Explain What Makes One Atom Different From Another Atom
An atom is a building block of matter that cannot be broken apart using any chemical means. Updated on August 15 2019.

Atoms To Molecules Earth Science
Inside the atom theres things called neutrons protons and electrons.

. An atom of gold is bigger and heavier. Explore the definition and parts of an atom and learn about devices that are used to measure the mass. Leaving behind the quantum physics an atom is made up of mainly electrons negatively charged and extremely light revolving around the nucleus protons positively charged heavy and sits in the nucleus and neutrons neutrally charged in the.
An atom is composed of two regions. An element is a substance in which all of the atoms have the same atomic proton number. Our current model of the atom can be broken down into three constituents parts protons neutron and electrons.
Unlike elements molecules can be made from the same or different elements. An atom is the smallest amount of a particular element that can be identified as that element. You can also have molecules of a single atom bonded together like two.
Protons and neutrons form the atomic nucleus. The neutrons and protons are all stuck together in the middle of the. Answer 1 of 8.
An atom is the smallest component of an element containing neutrons protons and electrons and makes up everything around us. Therefore to be precise atoms are the smallest part or amounts of elements. Due to this anomaly its place in periodic table is controversial.
Are the smallest bits of ordinary matter. Cut apart a single atom of iron and you will find 26 protons and 30 neutrons clumped together in the nucleus and 26 electrons whizzing around the outside. Well hydrogen is different from other elements in many ways.
In one way atoms are put together to form things called molecules. If three oxygen atoms bond together you get. For example water is a molecule made of hydrogen and oxygen.
Atoms form chemical bonds to make their outer electron shells more stable. So while an atom is its own separate entity a molecule is what you get when those atoms bond together. Simultaneouslyit can also lose one electron to form a cation.
The nucleus in the center is surrounded by clouds of electrons. The three parts of the atom are protons positively charged neutrons neutral charge and electrons negatively charged. Protons and neutrons form the nucleus of an atom and a cloud of electrons orbits the nucleus.
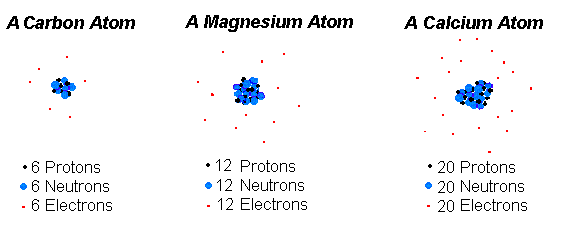
What makes one atom different from another. Each of these parts has an associated charge with protons carrying a positive. 9 Atoms of different elements with different mass numbers but same number of neutrons 10 Atoms of different elements having same atomic numbers DOWN 2 Atoms of same element having different mass number 3 Number of protons in an atoms 4 Proposed planetary model 6 Fundamental particle with no charge 7 Number of protons and neutrons.
Firstlyit needs one election to complete its duplet. Whereas an element is. These might be the same elements such as two oxygen atoms bonded together O2 or it might be different atoms bonded together like water H2O.
An element is a pure substance that consists entirely of one type of atom. A proton is a positively charged particle that resides in the nucleus the core of the atom of an atom and has a mass of 1 and a charge of 1. All atoms contain protons electrons and neutrons Figure 2.
The type of chemical bond maximizes the stability of the atoms that form it. An ionic bond where one atom essentially donates an electron to another forms when one atom becomes stable by losing its outer electrons and the other atoms become. Nuclear reactions can alter atoms.
To understand molecules you have to understand what an atom is made up of. The difference between one element and another stems from the number of protons and electrons in each atom. In their center atoms have a closely packed nucleus.
The difference between elements stems from their atomic structure. Its what makes one element different from another. It is also defined as a substance which cannot be broken down by chemical means.
These electrons are negatively-charged particles. The only exception is hydrogen H which is made of one proton and one electron. If an atom has a different number of electrons and protons it is called an ion.
Atoms bond with one another so that they can lower their energy and become stable. There are several ways that atoms can combine. The atom is the smallest unit of matter that forms and defines the structure of an element.
Lets use the model below to explain how atoms bond to become stable. What makes an atom of gold different from an atom of iron is the number of protons neutrons and electrons inside it. An atom is considered to be electrically neutral if it has an equal number of protons and electrons.
When the negatively charged atom anion and the positively charged atom cation. A substance that is made entirely from one type of atom. Atoms are made up of three subatomic particles.
Correct me if Im wrong. The number of protons in an atom is the defining feature of an atom. Neutrons like protons reside in the nucleus of an atom.
Secondarily in the number of neutrons in the. A molecule is made up of atoms bonded together. The key to a molecule is that two or more atoms are bonded together.
An important principle to know is electrons may be transferred from one atom to another or even shared between atoms allowing atoms to bind together. Answer 1 of 17. Electrons are attracted to the protons in the.
An atom is the smallest particle. Primarily the number of protons in the atoms nucleus which is the same as the number of electrons it has. Protons and neutrons have approximately the same mass about 167 10-24 grams which scientists define as one atomic mass unit amu or.
The number of protons in an atom is called its atomic number. What makes one element different from another is the number of protons in the nucleus of its atoms. The nucleus which is in the center of the atom and contains protons and neutrons and the outer region of the atom which holds its electrons in orbit around the nucleus.
Its actually made of two hydrogen atoms and one oxygen atom. And as atoms bond with other atoms they often make molecules with unique chemical and physical properties. Atoms themselves can be broken down into smaller sub-atomic particles protons neutrons and electrons - the amounts of these particularly the number of protons define each element.
Atoms are the simplest unit of a matter. This is the primary difference between an atom and element.
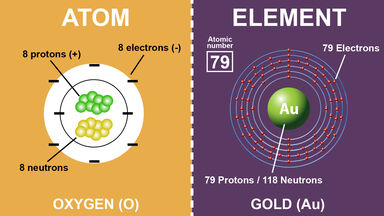
Difference Between Atoms And Elements With Examples

Atoms And The Periodic Table Activities Game For Learning Atomic Structure Middle School Science Classroom Teaching Chemistry Science Classroom

No comments for "Explain What Makes One Atom Different From Another Atom"
Post a Comment